Study selection
The database search identified 7709 studies. After deduplication, screening, and full-text assessment, 62 papers were included for data extraction (Fig. 1).
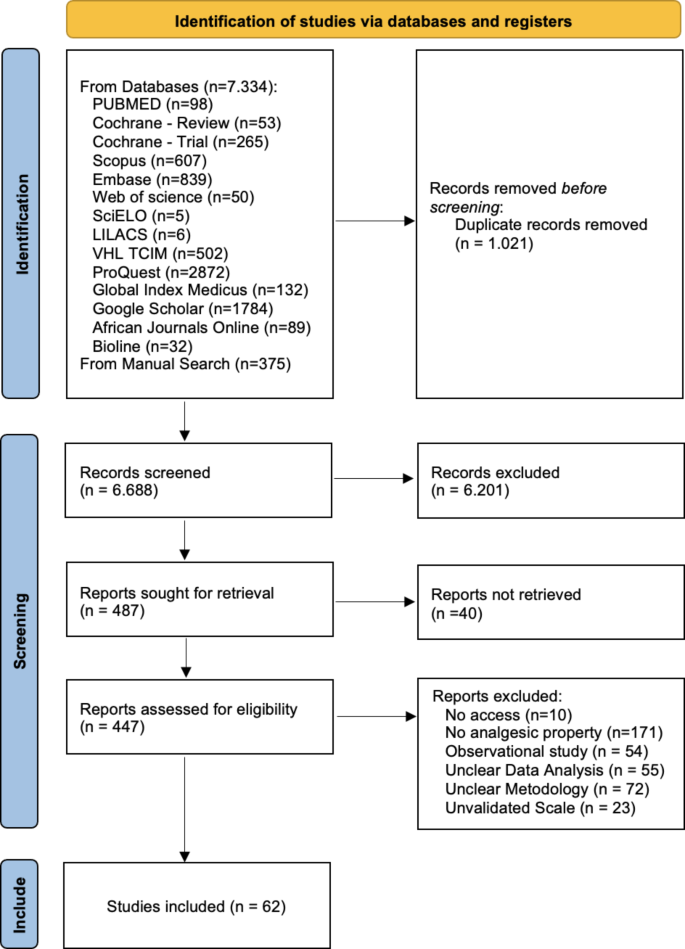
PRISMA flow diagram of the study selection.
Study characteristics
Most studies were blinded (n = 47), and the sample sizes ranged from 15 to 270 patients. The study characteristics are shown in Table 1; 70 different plants from 44 plant families were identified (Supplementary File).
Risk of bias
Regarding the risk of bias assessment, the majority of the studies had some concerns (n = 27), followed by those with low risk (n = 22) and high risk (n = 13). Six studies had a high risk in selection of the reported results17,18,19,20,21,22; 2, outcome measurement23,24; 2, missing outcome data19,25; 2, deviation from intended intervention26,27; and, 11, randomization process28,29,30,31,32,33,34,35,36,37,38 (Fig. 2).
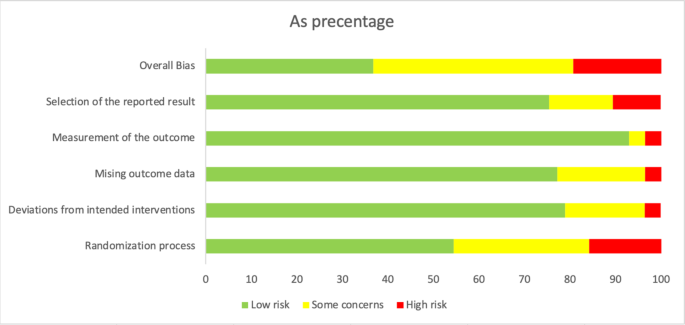
Risk of bias assessment of included studies.
Synthesis of results
The 62 papers identified 17 painful orofacial conditions, which were categorized into six distinct groups based on their origin: periodontal pain, endodontic pain, oral mucosal pain, oral neuropathic pain, oral surgery pain, and temporomandibular disorder (TMD) pain (Table 1).
Periodontal pain
The periodontal pain group included patients with five conditions: pericoronitis, free gingival graft, orthodontic pain, periodontal flap, and both surgical and non-surgical periodontal therapy.
In the study led by Shahakbari et al. (2014), the pain associated with pericoronitis notably reduced in 97 patients treated with green tea compared with that in those treated with 0.12% chlorhexidine. Similarly, Keceli et al., (2015) conducted a clinical trial involving 33 patients with free gingival grafts, where they noted a significant improvement in pain relief when a topical Ankaferd Blood Stopper was administered compared to placebo.
Based on a study of 80 patients experiencing orthodontic pain, Patil et al., (2018) revealed that belladonna exhibited superior analgesic properties compared to that of ibuprofen. Meanwhile, Das et al., (2019) clinical trial involving 20 patients with periodontal flap showed that those treated with Traumeel exhibited lower pain scores than those treated with ibuprofen. Additionally, in a study conducted by Anil et al., (2019) involving 15 patients (30 sites) with periodontal flaps, significant analgesic properties were observed for curcumin when compared to placebo.
Alshibani et al., (2022) examined the effects of ginger tablets in a cohort of 44 patients, whereas Al-Askar et al., (2022) administered curcumin capsules to 76 patients, all of whom had undergone periodontal therapy. In both investigations, no statistically significant differences were observed between the intervention and control groups which were given ibuprofen and mefenamic acid, respectively.
A meta-analysis of six studies18,23,33,34,39,40 revealed that green tea, Atropa belladonna, curcumin and ginger were more effective in reducing periodontal pain as compared to standard therapies (Fig. 3), and Ankaferd Blood Stopper and curcumin were more effective in reducing periodontal pain as compared to the placebo (Supplementary File). Network meta-analysis and ranking based on the probability of each treatment being the best were performed among the five interventions (Fig. 5). The surface under the cumulative ranking curve (SUCRA) of the treatment with Fabaceae combination and Solanaceae was 90.6% and 65.3%, respectively, confirming that these plant families are the top two best interventions for periodontal pain over Zingiberaceae (32.2%), NSAID’s (32.4%) and placebo (29.5%) interventions (Fig. 6).
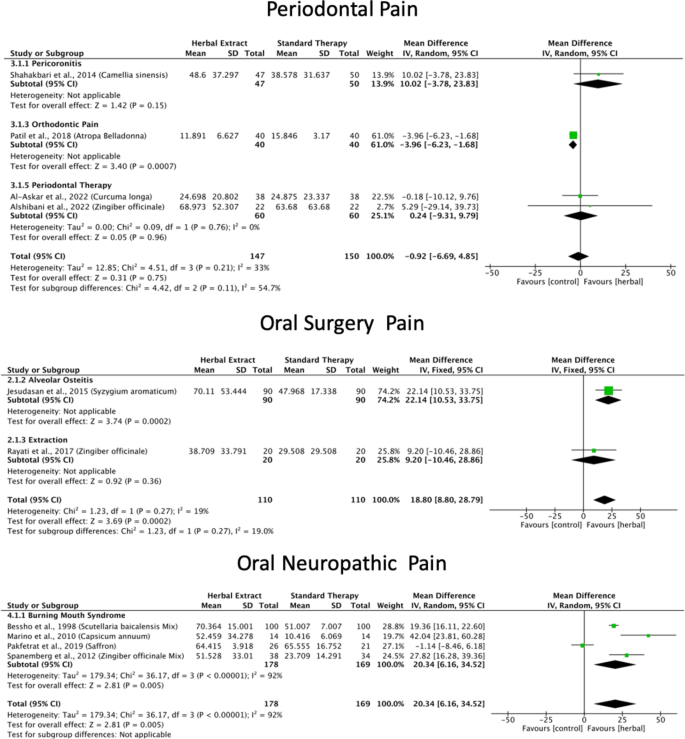
Herbal extracts for periodontal pain, oral surgery pain and oral neuropathic pain forest plots.
The other two studies could not be included in the meta-analysis owing to insufficient measurements over time25, and lack of transitivity18.
Oral surgery pain
Among the included studies, three conditions were identified: anesthesia infiltration pain, alveolar osteitis, and extraction.
Jesudasan et al. (2015) examined the efficacy of a eugenol paste derived from cloves in 270 patients with alveolar osteitis. Patients treated with eugenol experienced significantly greater relief in postoperative pain, inflammation, infection, and wound healing than that of those treated with 0.2% chlorhexidine gel. Various topical formulations have been investigated in clinical studies to address the pain caused by anesthetic infiltration. For instance, Alqareer et al. (2006) evaluated a gel containing clove in 73 patients, whereas Mohite et al. (2020) used interventions with Anacyclus pyrethrum and Spilanthes acmella gels in 30 patients. In both studies, no statistically significant differences in pain were found in comparison with the control groups (benzocaine and lignocaine gels).
In a clinical trial involving 60 patients41, ginger powder proved to be as effective as ibuprofen for managing postsurgical sequelae after extraction. In a study conducted by Komasawa et al. (2018) involving 60 patients, the researchers investigated the efficacy of preoperative administration of Jidabokuippo, a combination of botanical extracts, including Cinnamomi cassiae, Clove, Licorice, Ligusticum wallichii, Nuphar japonica, Quercus robur, and Rheum rhabarbarum. They compared the effects of Jidabokuippo in the management of pain after tooth extraction with those of a no-treatment group. The study’s findings revealed that the severity of postoperative pain was significantly reduced in the Jidabokuippo group at 3 and 24 h after anesthesia recovery.
A homeopathic medicine known as Traumeel (that containing Arnica montana, Calendula, Chamomile, St. John’s wort, Aconitum napellus, Bellis perennis, Atropa Belladonna, Echinacea purpurea, Echinacea angustifolia, Hamamelis virginiana, Achillea millefolium, Symphytum officinale) has been used for pain, edema, and trismus relief after third molar surgery in 17 patients22, suggesting that Traumeel might be a good alternative which is comparable to dexamethasone.
The meta-analysis of the three studies41,42,43 found that clove and ginger were more effective in reducing oral surgery pain than standard therapies (Fig. 3), and Jidabokuippo, clove and ginger were more effective in reducing oral surgery pain than the negative control or placebo (Supplementary File). The network meta-analysis and ranking based on the probability of each treatment being the best were performed for the six interventions (Fig. 5). The SUCRA of the treatment with Myrtaceae and Zingiberaceae was 98% and 80.6%, respectively, confirming that these two plant families are the best interventions for oral surgical pain over chlorhexidine (61.4%), Myrtaceae combination (20%), and placebo/no-treatment (0%) interventions (Fig. 6). The other three studies could not be included in the meta-analysis because of insufficient measurements over time21,44, and data not being expressed as mean and standard deviation22.
Oral neuropathic pain
Two conditions were identified in the studies included in the oral neuropathic pain group: burning mouth syndrome (BMS) and facial pain and sensitivity to mechanical, cold, and heat stimuli.
Marino et al. (2010) conducted a study of 56 individuals diagnosed with BMS who were treated with capsaicin, alpha-lipoic acid, lysozyme-lactoperoxidase, or boric acid. The results revealed a significant symptom score reduction in patients treated with capsaicin, alpha-lipoic acid, and lysozyme-lactoperoxidase, showing higher effectiveness than boric acid treatment. Spanemberg et al. (2012) examined the impact of Catuama, a herbal treatment containing Paullinia cupana, Trichilia catigua, ginger, and Ptychopetalum olacoides, in 72 patients with BMS. The results revealed a notable enhancement in the test group compared to magnesium silicate after a 4-week treatment period. Furthermore, this considerable improvement persisted even 12 weeks after treatment initiation.
Pakfetrat et al. (2019) conducted a clinical trial involving 47 patients with BMS treated with crocin, a herbal extract derived from saffron. The results of an 11-week trial demonstrated that crocin significantly decreased the severity of BMS symptoms, comparable to the effects of citalopram. In a study involving 200 patients, Bessho et al. (1998) employed sai-boku-to, a herbal extract derived from Bupleurum chinense, Pinellia ternata, Scutellaria baicalensis, Magnoliae Officinalis, Ziziphus mauritiana, Panax ginseng, licorice, Perilla frutescens, and ginger, for treating BMS. The results demonstrated that sai-boku-to exhibited effectiveness comparable to Diazepam + Vitamin B in reducing pain, burning sensations, and discomfort.
In a clinical trial conducted by Lee et al. (2007), the application of capsaicin to the facial skin of 40 patients resulted in decreased sensitivity to mechanical, heat, and cold-induced pain. Interestingly, this reduction in pain sensitivity occurred without affecting non-painful tactile sensations, as evidenced by a comparison with the pain sensitivity in the control group that did not receive capsaicin treatment.
According to the comprehensive meta-analysis of the five studies24,45,46,47,48, Catuama, crocin, Sai-boku-to, and capsaicin exhibited greater efficacy in reducing oral neuropathic pain compared to conventional treatment methods (Fig. 3). Moreover, capsaicin showed superior effectiveness in alleviating facial pain compared to that of the negative control (Supplementary File). Network meta-analysis and the ranking based on the probability of each treatment being the best was performed among the six interventions (Fig. 5). The SUCRA of the treatment with Solanaceae was 97.4%; Zingiberaceae combination, 75.6%; and Iridaceae, 56.5%, confirming that these three plant families are the best interventions for oral neuropathic pain over standard treatment (47.1%), Apiaceae combination (13.3%) and no-treatment (10.1%) interventions (Fig. 6).
Endodontic pain
The included studies focused on dentinal hypersensitivity (DH) and used tactile and air stimuli to measure DH levels.
In a study conducted by Majji and Murthy (2016), 160 patients were divided into four groups, each assigned to a different type of desensitizing toothpaste. The toothpaste formulations were evaluated. The findings indicated that all four toothpaste types (5% potassium nitrate, 5% CSPS (NovaMin), 10% strontium chloride, and a herbal formulation containing Calendula and Plantago major), effectively relieved dentinal hypersensitivity. Notably, the CSPS group demonstrated the most favorable clinical response at the end of the two-month period.
On the other hand, Kar et al. (2019) conducted a study with 45 adults, dividing them into three groups, each using a different type of toothpaste: potassium salt, 8% arginine, or a herbal desensitizing paste containing Spinacia oleracea, Clove, Terminalia chebula, Terminalia bellirica, and Phyllanthus emblica. The results of this study showed that the herbal toothpaste was more effective than the potassium nitrate-containing toothpaste in reducing dentinal hypersensitivity. However, the toothpaste containing 8% arginine was found to be the most effective in reducing DH.
According to the meta-analysis conducted in the two studies20,49, the standard therapies, typically found in commercially available desensitizing toothpastes, proved to be more effective in reducing endodontic pain than the Calendula, plantago, palakya, lavanga, and triphala toothpastes (Fig. 4). Network analysis and the ranking based on the probability of each treatment being the best were performed for the three interventions (Fig. 5). The SUCRA of the standard treatment was 88.3%, and for Asteraceae was 58%, confirming that these two treatments were the best interventions for endodontic pain over the Combretaceae combination (3.6%) intervention (Fig. 6).

Herbal extracts for endodontic pain, oral mucosal pain and TMD pain forest plots.
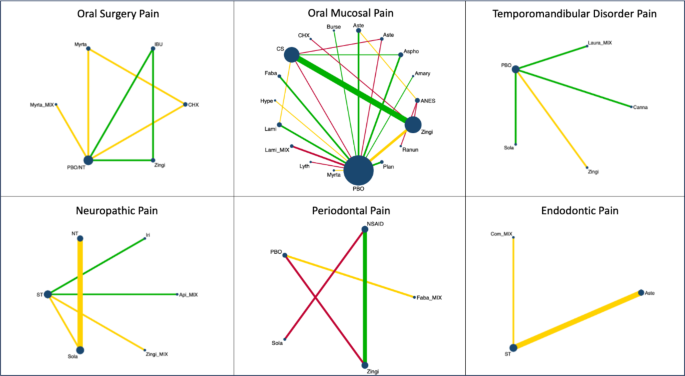
Herbal extracts for orofacial pain network plots categorized by type of orofacial pain and plant family.
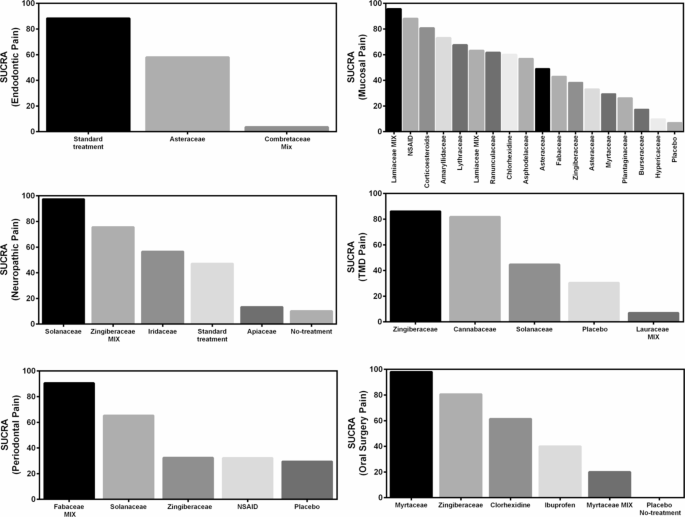
Surface under the cumulative ranking score (SUCRA) of herbal extracts for periodontal pain, oral surgery pain, oral neuropathic pain, endodontic pain, oral mucosal pain and TMD pain.
Oral mucosal pain
Out of the 38 studies included into the oral mucosal pain category, five conditions were identified: aphthous ulcers, oral mucositis induced by chemotherapy and/or radiotherapy, oral submucous fibrosis, oral lichen planus, and oral mucosal wounds resulting from orthodontic treatment.
Numerous clinical trials have investigated diverse herbal topical formulations to alleviate pain and discomfort associated with aphthous ulcers. For instance, the effects of turmeric have been examined by Deshmukh and Bagewadi (2014), Kia et al. (2020a), and Halim et al. (2013), whereas those of chamomile have been studied by Tadbir et al. (2015). These studies found no statistically significant differences in the alleviation of pain when compared with triamcinolone. Conversely, other studies have compared multiple herbal extracts, such as myrrh50, Hypericum perforatum51, allicin26, camel thorn52, Aloe vera53, pudilan30,54, Punica granatum29, and myrtle55. These studies found significantly better analgesic properties compared with placebos.
Curcumin’s efficacy as a pain-relieving remedy for patients with oral lichen planus was studied by Thomas et al. (2017), Kia et al. (2020b), Keshari et al. (2015), and Chainani-Wu et al. (2012). The results demonstrated significant analgesic properties, in comparison to triamcinolone, prednisolone, and placebo. Furthermore, the analgesic attributes of patients with oral lichen planus treated with chamomile56 or Aloe vera57 where compared with those treated with placebo and triamcinolone, respectively.
Multiple herbal extracts have been studied for the treatment of oral mucositis caused by chemotherapy and/or radiotherapy. For instance, significant analgesic properties have been reported for Plantago ovata58, Zataria multiflora59, Plantago mayor60, curcumin61, Aloe vera36, Salvia officinalis62 and licorice19 when compared with placebo. Other studies have found pain-relieving properties of chamomile63,64, curcumin35,65, Nigella sativa37, and chinning decoctions38 when compared to standard therapies.
The efficacy of curcumin as an analgesic has been studied in patients with oral submucous fibrosis27,66,67,68 when compared to placebo or standard therapies. Jiang et al. (2013) reported the analgesic properties of salvianolic acid in pain related to oral submucous fibrosis. In contrasts, Liu et al. (2022) found that licorice exerts analgesic effects on oral mucosal wounds resulting from orthodontic treatment.
According to a comprehensive meta-analysis of 36 studies, Curcuma longa, chamomile, Aloe vera, Nigella sativa, chinning decoction, Salvia miltiorrhiza, and clove exhibited greater efficacy in reducing oral mucosal pain than that of standard therapies (Fig. 4). Moreover, licorice, myrtle, Aloe vera, Punica grantum, allicin, pudilan, myrrh, St. John’s wort, camel thorn, chamomile, Zataria multiflora, Plantago ovata, Curcuma longa, Salvia officinalis and Plantago major had superior effectiveness in alleviating oral mucosal pain in comparison to placebo (Supplementary File).
The network meta-analysis and the ranking based on the probability of each treatment being the best were performed for the 18 interventions (Fig. 5). The SUCRA of the treatment with Lamiaceae was 95.6%; anesthetics, 88.1%; corticoiesteroids, 80.7; Amaryllidaceae, 73.1%; Lythraceae, 67.5%; Lamiaceae combination, 63.2%; Ranunculaceae, 61.6%; and, chlorhexidine, 60%, confirming that these are the best eigth interventions for oral mucosal pain over Asphodelaceae (57%), Asteraceae (49%), Fabaceae (43%), Zingiberaceae (38.3%), Asteraceae (33.2%), Myrtaceae (29.4%), Plantaginaceae (26.3%), Burseraceae (17.4%), Hypericaceae (9.8%), and placebo (7%) interventions (Fig. 6). Two studies were not included in the meta-analysis because the data were not expressed as mean and standard deviation32,67.
Temporomandibular disorder pain
Four studies about TMD pain were included. In a clinical trial by Li et al. (2009), 55 subjects with temporomandibular joint (TMJ) pain receivied Ping On ointment containing Mentha piperita, Cinnamomum camphora, Gaultheria fragrantissima, Santalum album, and eucalyptus or a placebo for 4 weeks. Patients reported that Ping On ointment significantly reduced the painful symptoms of the TMJs, and they felt more comfortable opening their mouths than the placebo group. In another study by Campbell et al. (2016), 15 patients with TMD were treated with a high-concentration capsaicin (8%) cream or placebo for a week, and the results showed a significantly higher pain-relief response in the week after application in the capsaicin-treated subjects with TMD.
Chaimano et al. (2021) showed that the subjects with myogenic TMD pain who underwent pain treatment with a herbal compress ball, containing Cassumunar ginger, turmeric, and camphor, had greater pain-free maximum opening compared to those who only used the warm placebo. Nitecka-Buchta et al. (2019) investigated the myorelaxant properties of cannabidiol (CBD) administered topically to the masseter muscle of 60 patients who experiencied myofascial pain. The results revealed a significant reduction of 70.2% in pain intensity in the CBD-treated group as compared to that in the placebo group which exhibited only a 9.81% reduction. Moreover, CBD application led to decreased activity and enhanced condition of the masticatory muscles.
The comprehensive meta-analysis of all four studies69,70,71,72 revealed that capsaicin, cannabis, Ping on ointment and cassumunar ginger, turmeric, and camphor exhibit greater efficacy in diminishing TMD pain compared to placebo (Fig. 4). Network meta-analysis and the ranking based on the probability of each treatment being the best were performed for the 5 interventions (Fig. 5). The SUCRA of the treatment with Zingiberaceae was 86.1%, and that for Cannabaceae was 81.8%, confirming that these two treatments are the best interventions for TMD pain over the Solanaceae (44.7%), placebo (30.4%), and Lauraceae combination (7%) interventions (Fig. 6) (Table 2).
link

